Assessing partition composition differences¶
First made as a blurb 2010-11-13
Revised for Sphinx 2016-05-30
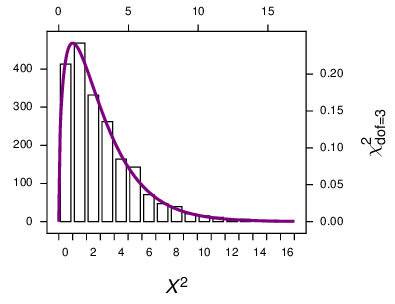
Here we ask whether different data partitions of molecular sequence alignments have different compositions — a deceptively simple question. It needs to be answered using a model of evolution.
I use the \(X^2\) statistic because it usefully reflects the heterogeneity in the data. However I find that I cannot validly use the \(\chi^2\) curve as a null distribution to assess the significance of that \(X^2\). Rather I need to make a null distribution using simulations, and making these null distributions is what this explanation is about. (Here I make a distinction between the \(X^2\) statistic, using a capital \(\chi\), calculated from an \(R \times C\) table, and the \(\chi^2\) curve that is used to assess its significance. I got that from Sokal and Rohlf.)
Comparing constant with variable sites¶
Here the model, the tree, and data size that I use is the same as that above except that it includes a 4-category gamma distributed among site rate variation with shape of 0.5. When I simulate data of 200 characters with that, the number of constant sites in the resulting alignment ranges from about 50 to about 90, as shown in Figure 8.
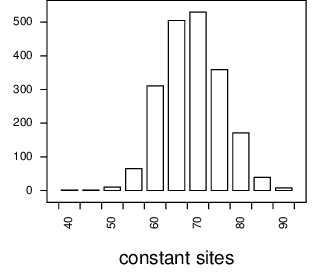
Figure 8 Distribution of the number of constant sites. For each of 2000 replicates, 11 DNA sequences of length 200 were simulated on a star tree, and the number of constant sites counted and plotted.¶
How can we make a valid null distribution? The data for which we want to make the comparison will have a certain number of constant sites, \(N_C\). We want to make a valid null distribution with our tree, model, and data size, that would be appropriate for comparing the composition of the constant vs the variable sites of our alignment with the \(N_C\) constant sites. Here I test getting \(X^2\) values from simulations with 3 different regimes,
- Regime 1
Do many simulations but only use the simulations where the number of constant sites in the simulation is equal to \(N_C\). Here I arbitrarily chose \(N_C = 71\).
- Regime 2
Use all the simulations, comparing constant sites with variable sites
- Regime 3
Use all simulations, without distinguishing between constant and variable sites. Compare the first half of the alignment with the second half.
As shown in Figure 9, distributions from all three regimes all have similar distributions. However, while the Regime 1 and 2 distributions (red and orange bars, respectively) appear to be about the same as each other, it appears that the Regime 3 distribution (blue bars) is slightly different.
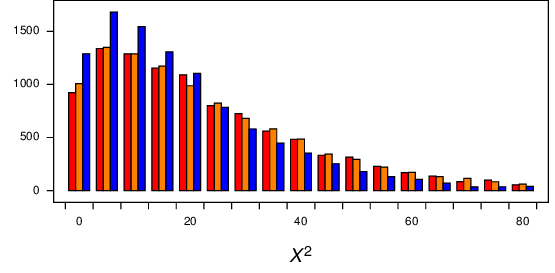
Figure 9 Distribution of \(X^2\) from simulations under regimes 1 (red bars), 2 (orange bars), and 3 (blue bars). Alignment length was 200. All three distributions have a long tail (up to about 180), which has been truncated here.¶
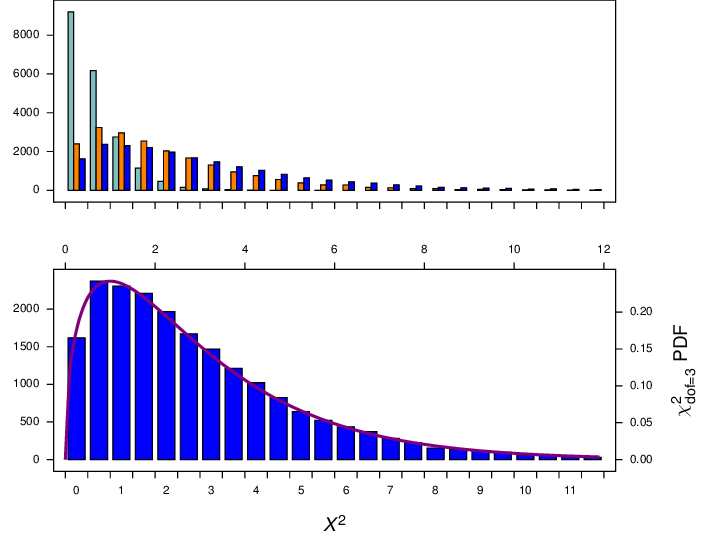
Thinking that the explanation for this difference was because the alignment was so short, I repeated the simulations with a long, 10000 - base alignment (for regimes 2 and 3; regime 1 was too slow). However, I got the same difference, as shown in Figure 10 (orange bars vs blue bars), so the length of the sequences does not appear to affect things. Thinking that the explanation might be that the two partitions compared might be different sizes (in regime 2 about 3500 of the 10000 sites were constant, while in regime 3 I compared equal sized partitions) I modified regime 3 for another distribution where I compared the first 3000 sites with the remaining 7000 sites in the alignment. That distribution is shown in the light blue bars in Figure 10. It appears that makes no difference as well.
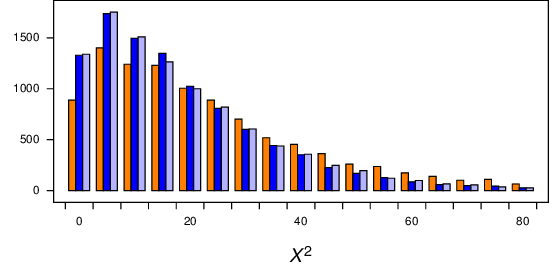
Figure 10 Distribution of \(X^2\) from simulations of long alignments (10000 characters) under regimes 2 (orange bars), and 3 (blue bars), and a modified regime 3 wheter the first 3000 positions was compared with the remaining 7000 positions(light blue). All three distributions have a long tail (up to about 180), which has been truncated here.¶
Comparing autapomorphic sites with other variable sites¶
Autapomorphic sites are sites with two different character states, where one character state appears only once; that is a constant site with a single character different. If we simulate data sets as above (star tree, 200 characters long), we get a distribution of autapomorphiec sites is as shown in Figure 11.

Figure 11 Distribution of the number of autapomorphic sites when data were simulated on the star tree with among-site rate variation (see text).¶
We want to get a valid null distribution by which to evaluate any difference in composition between autapomorphic sites and the other variable sites. As above, 3 different simulation regimes were done, as follows, where \(N_A\) is the number of autapomorphies in the original data, which I will arbitrarily set to 30.
- Regime 1
Do many simulations but only use the simulations where the number of autapomorphies in the simulation is equal to \(N_A\).
- Regime 2
Use all the simulations, comparing autapomorphic sites to all other variable sites
- Regime 3
Use all simulations, without distinguishing autapomorphies. Compare the first half of the variable sites with the second half.
Results for these simulations are shown in Figure 12. The distributions for the 3 regimes appear to be similar. The fourth set of simulations are \(X^2\) values from comparing the first half of the data with the second half, without partitioning into constant sites or singletons. Even that distribution is similar to the others.
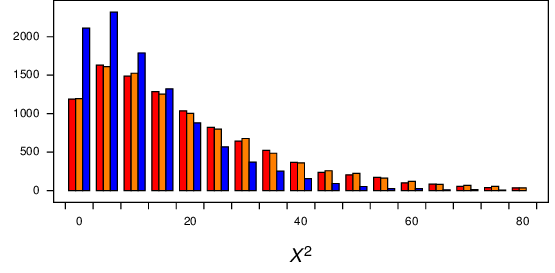
Figure 12 Comparison of null distributions of \(X^2\) for comparisons of autapomorphic sites with other variable sites. The red bars show regime 1, the orange bars show regime 2, and the blue bars show regime 3.¶
It looks like the distributions for the first two regimes are about the same, while the distribution for regime 3 differs.
An example using the Cox et al 2008 data¶
Here I use the Cox *et al* 2008 SSU and LSU rRNA gene alignment, including all sites (Cox et al stripped out constant sites and autapomorphies for the published analysis), and doing it in a Bayesian context. A Bayesian run was made using MrBayes v3.2.2, for 2 million generations, using the GTR+IG model on the two partitions, unlinked. The two runs appeared to converge well, with the ASDOSF about 0.01 – 0.02, and PSRF values close to 1.0. The second half of the samples were used for simulations to get null distributions by which to compare the constant vs variable, and autapomorphies vs other variable sites for both partitions. Samples from both runs were used. (The consensus tree was the 3-domains tree). Results are shown in Table 1, and show that for both genes the composition of the constant sites differs (at the 5% level) from that of the variable sites. The composition of autapomorphies differs from the other variable sites in the LSU gene, but not in the SSU gene.
The simulations were done as described in this listing, saving the xSq values in a file called xSq.
# Read in a tree with an attached model, as a pickled tree
read('myTree.p4_tPickle')
t = var.trees[0]
# Read in some data.
var.alignments = []
var.doCheckForBlankSequences=False
var.doCheckForDuplicateSequences=False
read('ssu.nex')
read('lsu.nex')
aSsu = var.alignments[0]
aLsu = var.alignments[1]
d = Data()
# Check to make sure its all good to go.
# (This step is needed for empirical comps, at least)
t.data = d
t.calcLogLike(verbose=False)
for myRunNum in [1,2]:
# Instantiate
ps = PosteriorSamples(t, runNum=myRunNum, program='mrbayes', mbBaseName='mbout', verbose=0)
# # Iterate over samples
for sampNum in range(1001,2001):
t2 = ps.getSample(sampNum)
t2.data = d
t2.simulate()
for a in [aSsu, aLsu]:
mC = a.constantMask()
mS = a.getMaskForAutapomorphies()
mOV = a.orMasks(mC, mS)
aC = a.subsetUsingMask(mC)
aV = a.subsetUsingMask(mC, inverse=True)
aS = a.subsetUsingMask(mS)
aOV = a.subsetUsingMask(mOV, inverse=True)
#print aOV.getCharDiversityDistribution()
# Count characters in the four subsets, make xSq
cC = [0] * 4
for s in aC.sequences:
cc = [s.sequence.count(symb) for symb in aSsu.symbols]
for i in range(aSsu.dim):
cC[i] += cc[i]
cV = [0] * 4
for s in aV.sequences:
cc = [s.sequence.count(symb) for symb in aSsu.symbols]
for i in range(aSsu.dim):
cV[i] += cc[i]
rByC = [cC, cV]
xSq = func.xSquared(rByC)
print xSq,
cS = [0] * 4
for s in aS.sequences:
cc = [s.sequence.count(symb) for symb in aSsu.symbols]
for i in range(aSsu.dim):
cS[i] += cc[i]
cOV = [0] * 4
for s in aOV.sequences:
cc = [s.sequence.count(symb) for symb in aSsu.symbols]
for i in range(aSsu.dim):
cOV[i] += cc[i]
rByC = [cS, cOV]
xSq = func.xSquared(rByC)
print xSq,
print
The \(X^2\) for the original data was made as shown here, and assessed using the null distributions in the four columns in the xSq file.
from p4 import *
# Read in some data.
var.alignments = []
var.doCheckForBlankSequences=False
var.doCheckForDuplicateSequences=False
read('A_mb/ssu.nex')
read('A_mb/lsu.nex')
aSsu = var.alignments[0]
aLsu = var.alignments[1]
d = Data()
fourStats = []
for a in [aSsu, aLsu]:
mC = a.constantMask()
mS = a.getMaskForAutapomorphies()
mOV = a.orMasks(mC, mS)
aC = a.subsetUsingMask(mC)
aV = a.subsetUsingMask(mC, inverse=True)
aS = a.subsetUsingMask(mS)
aOV = a.subsetUsingMask(mOV, inverse=True)
# Count characters in the four subsets, make xSq
cC = [0] * 4
for s in aC.sequences:
cc = [s.sequence.count(symb) for symb in aSsu.symbols]
for i in range(aSsu.dim):
cC[i] += cc[i]
cV = [0] * 4
for s in aV.sequences:
cc = [s.sequence.count(symb) for symb in aSsu.symbols]
for i in range(aSsu.dim):
cV[i] += cc[i]
rByC = [cC, cV]
xSq = func.xSquared(rByC)
print xSq,
fourStats.append(xSq)
cS = [0] * 4
for s in aS.sequences:
cc = [s.sequence.count(symb) for symb in aSsu.symbols]
for i in range(aSsu.dim):
cS[i] += cc[i]
cOV = [0] * 4
for s in aOV.sequences:
cc = [s.sequence.count(symb) for symb in aSsu.symbols]
for i in range(aSsu.dim):
cOV[i] += cc[i]
rByC = [cS, cOV]
xSq = func.xSquared(rByC)
print xSq,
fourStats.append(xSq)
print
for myCol in range(4):
myStat = fourStats[myCol]
n = Numbers('A_mb/xSq', col=myCol)
n.tailAreaProbability(myStat)
print
830.616014485 125.478358423 911.356762349 478.645980872
# The stat is 830.616014485
# The distribution has 2000 items
# The distribution goes from 0.143587053251 to 1295.68793501
# Items in the distribution were >= theStat 7 times.
# The tail-area probability is 0.003500
# The stat is 125.478358423
# The distribution has 2000 items
# The distribution goes from 0.188236493375 to 807.554343692
# Items in the distribution were >= theStat 896 times.
# The tail-area probability is 0.448000
# The stat is 911.356762349
# The distribution has 2000 items
# The distribution goes from 0.602332757867 to 1044.1673624
# Items in the distribution were >= theStat 4 times.
# The tail-area probability is 0.002000
# The stat is 478.645980872
# The distribution has 2000 items
# The distribution goes from 1.36834386638 to 1124.15527494
# Items in the distribution were >= theStat 71 times.
# The tail-area probability is 0.035500
gen |
comparison |
tail-area probability |
|---|---|---|
SSU |
constant vs variable |
0.004 |
autapomorphies vs other variable |
0.448 |
|
LSU |
constant vs variable |
0.002 |
autapomorphies vs other variable |
0.036 |